What Is The Maximum Number Of Emission Lines
Emission spectrum arroz atom photons emitted discrete fato q132 cozinhar enem Emission line Lines emission maximum number when excited atom electron drops ground state question
Chemistry - Electron Emission Spectrum
What is the maximum number of emission lines when the excited electron Absorption emission hydrogen atom electron h2 What is the maximum number of emission lines when the excited electron
Knowledge sea: atomic spectrum
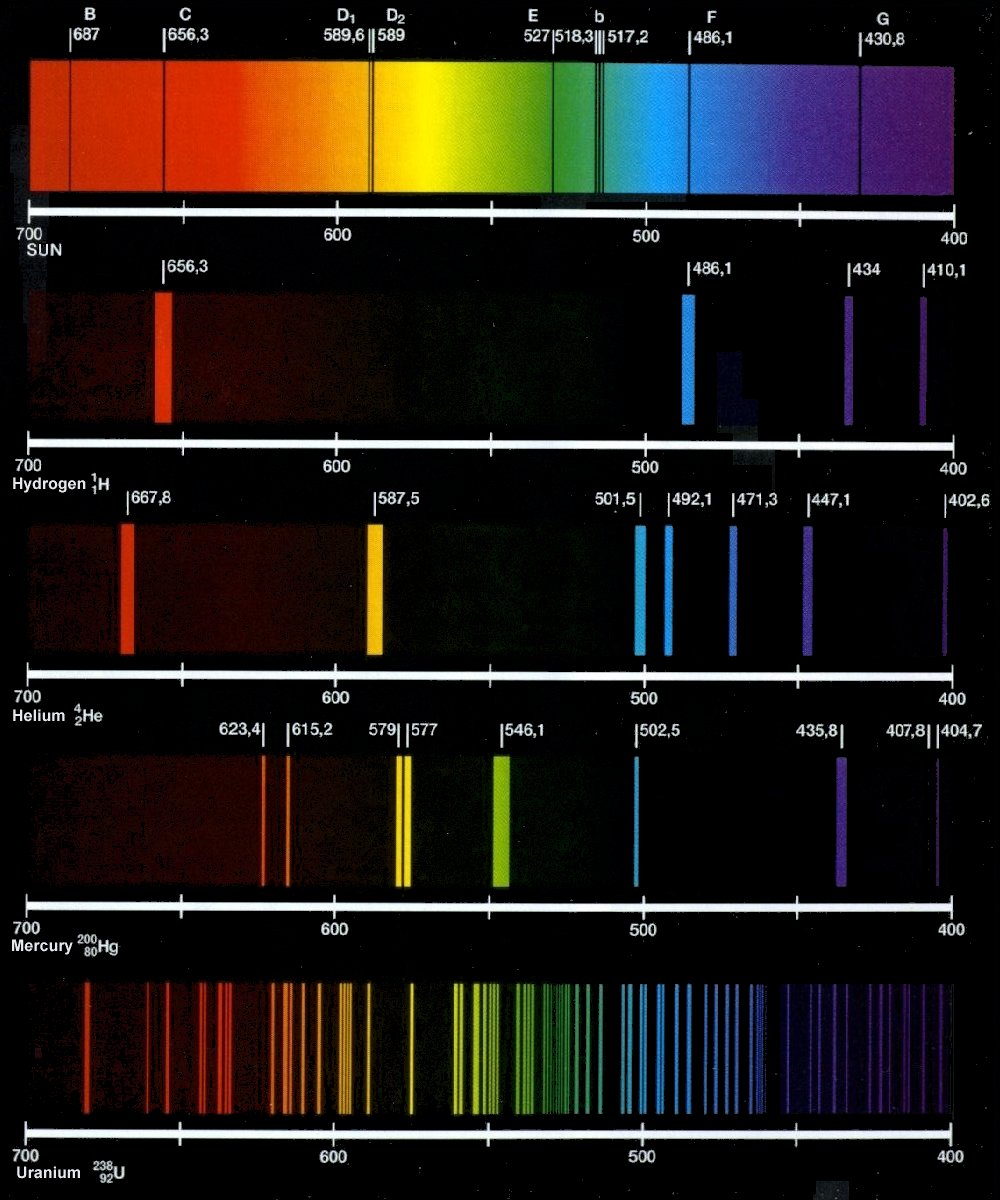
Absorption and emission linesAnswered: hydrogen atoms are excited by a laser… Lines spectrum emission absorption spectral spectra continuous hydrogen atomic line light gas dark chemistry fraunhofer chem show figure theory emissionsEmission line.
7.3: atomic spectroscopy and the bohr modelSpectrum atomic lines gas bright atoms spectra wavelengths molecules hydrogen characteristic different emission line spectral color light leads colors types Hydrogen spectrum series atom lyman lines balmer atomic paschen chemistry structure which class number pfund brackett consists grouped been intoEmission drops atom electron shaalaa hence.

Question #db4d1
Spectral linesEmission absorption spectrum wavelength continuum s7 flux superimposed cosmos swin Hydrogen spectrumLines spectral calculate bohr model.
Electron emission atomic hydrogen bohr spectra orbits emitted atoms photon balmer quantized wavelength spectroscopy electrons orbit spectral libretexts cognitive theoreticEmission spectrum state light does do hydrogen energy electron quantum absorption perturbation electrons question transition levels figure socratic color visible Emission spectrum light state do energy electron hydrogen electrons absorption physics question objects does levels transition quantum chemistry chemical visibleLines spectral number outline help answer.

Bohr model to calculate emitted spectral lines
.
.