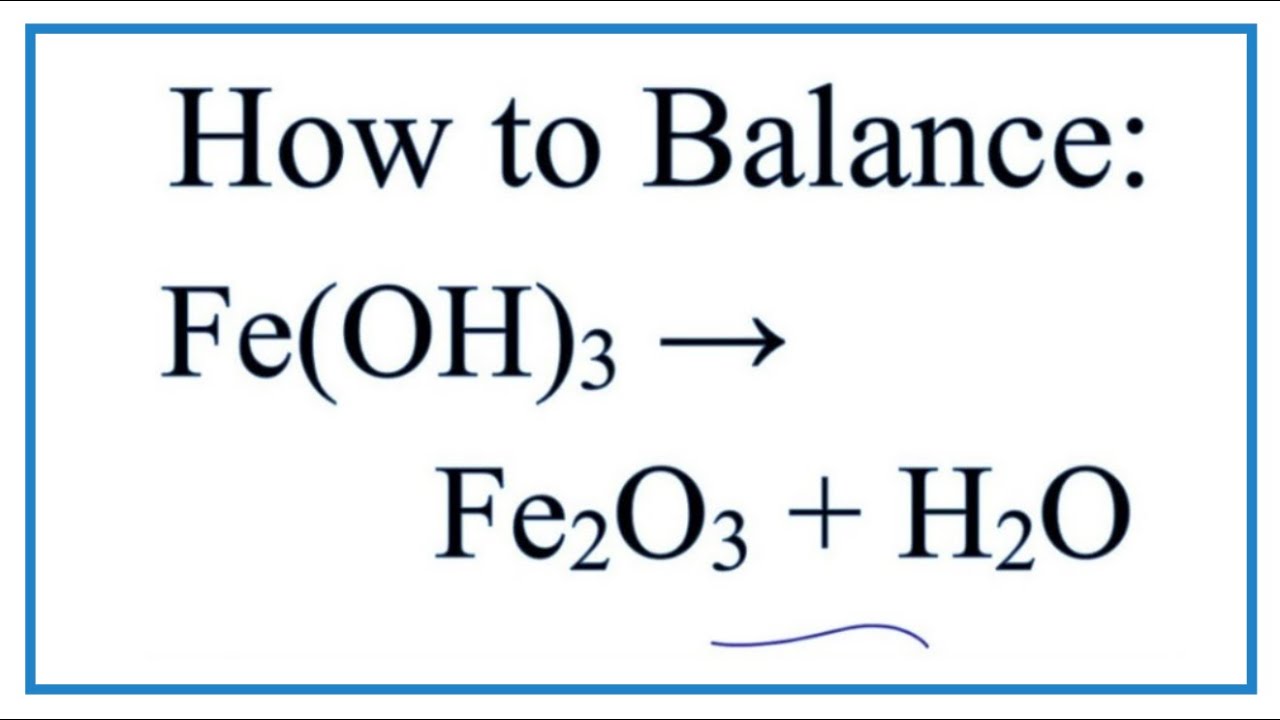
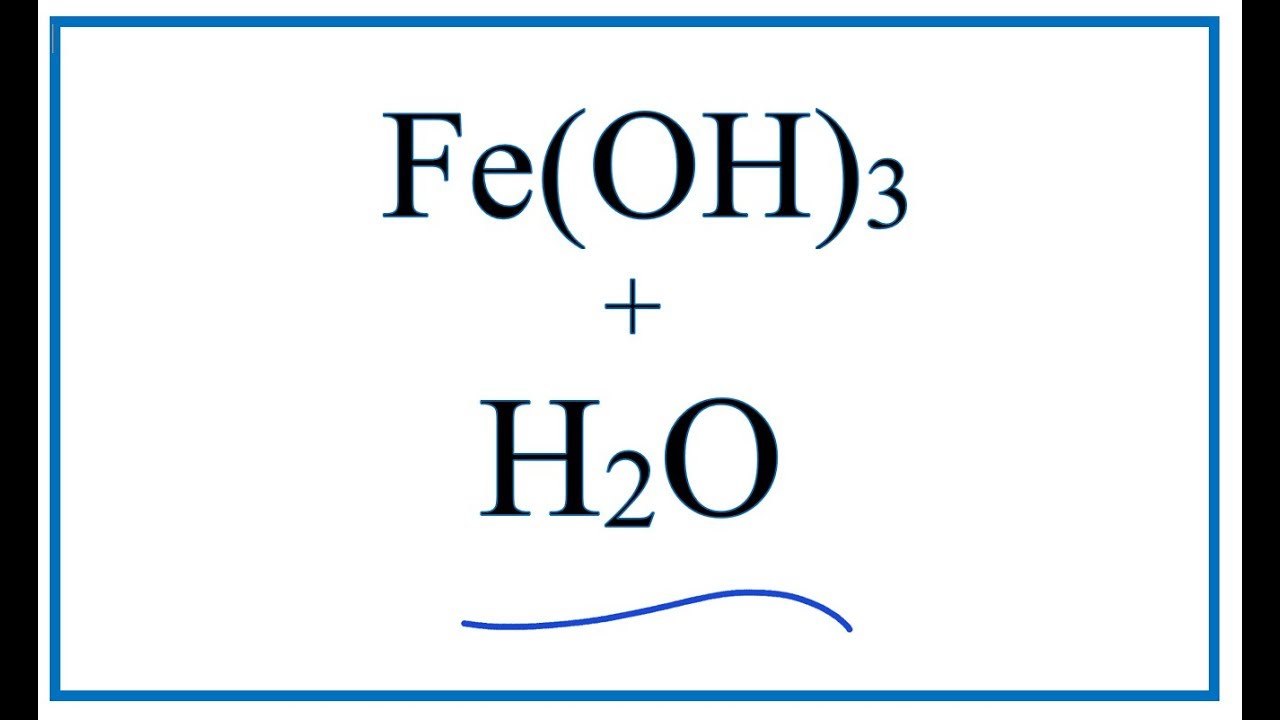
What Is Fe Oh 3
Oh fe name Fe oh del equilibrio How to write the name for fe(oh)3
Chemical reaction-Rusty red iron(III) hydroxide precipitate (Fe(OH)3
Equation for fe(oh)3 + h2o Fe2o3·nh2o feo(oh)·fe(oh)3 on behance Chemical reaction-rusty red iron(iii) hydroxide precipitate (fe(oh)3
Ferric oh fe hydroxide precipitation precipitate fe2 sodium h2o so4 naoh beaker fphoto photoshelter
Fe hydroxide oh iron iii h2o water equationFe oh fecl3 naoh Formation of fe(oh)3 after adding naoh to fecl3Science chemistry precipitation reaction ferric oxide.
Solved consider the following reactions taking place:How to balance fe(oh)3 and heat = fe2o3 + h2o Pourbaix considering substancesFeoh hydroxide iii iron.

Pourbaix diagram of iron considering fe(oh)2 and fe(oh)3 as solid
Fe hydroxide oh structure chemical iron nameFe oh iron hydroxide iii h2o decomposition heat balance Oh fe2 h2o consider reactions chegg answersType of reaction for fe(oh)3 = fe2o3 + h2o.
Feo nh2oPourbaix considering silicon substances Fe oh hydroxide iron xrd nanopowder nanoparticles ray coatedIron iii hydroxide reaction chemical fe fecl3 tube nacl oh h2o test precipitate carbonate red co2 chloride rusty sodium formed.

Pourbaix diagram of iron considering fe(oh)2 and fe(oh)3 as solid
H2o ch3coohIron hydroxide (fe(oh)3)|1309-33-7,angenechemical Iron hydroxide nanopowderEquilibrio de precipitación del fe(oh)3.
.